Our research group of catalytic chemistry research group (Group 501) Shen Wenjie and other researchers from the Shenyang Institute of Metals, Chinese Academy of Sciences, have made important progress in the study of the morphological effects of iron oxide nanomaterials. The latest research results are published on Angew. Chem. Int. Ed.
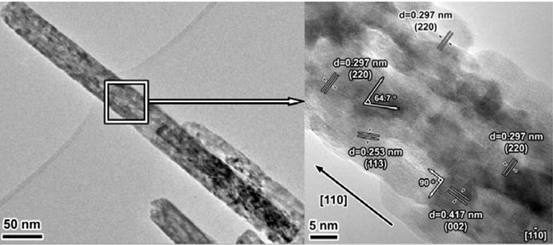
In this work, the dehydration method of the β-FeOOH precursor was modified by solvent heat treatment, and the γ-Fe2O3 nanorod with high temperature stability was obtained for the first time.
Through the control of crystal phase and morphology, the material selectively exposes {110} and {001} crystal faces, which are rich in both Fe and O atoms, which is beneficial to the efficiency of NO and NH3 molecules in an oxygen-rich atmosphere. activation. It exhibits a wide temperature window (200-400 ° C, 80% NO conversion) and strong resistance to water and sulfur in the selective catalytic reduction of ammonia (NH3-SCR). It is expected to be the main active group. It plays an important role in the development of new and efficient DeNOx catalysts.
In recent years, the catalytic reaction chemistry research group has carried out research on the structure and effect control of nanomaterials and the structure-activity relationship of catalysts. Recently, the structurally regular Co3O4 nanorods have been successfully prepared, and the surface active sites are used to catalyze the low temperature of carbon monoxide. Oxidation (Nature, 458 (2009) 746-749). These results have important theoretical significance and application value for the development of high activity metal oxide catalysts. (Text by Chuanchuan Jin)