Our group in collaboration with Prof. Jiahui Huang group (2301), made new progress in explaining the size effect of gold nanocatalysts and the active site of CO oxidation reaction. The finding was published in Small Methods.
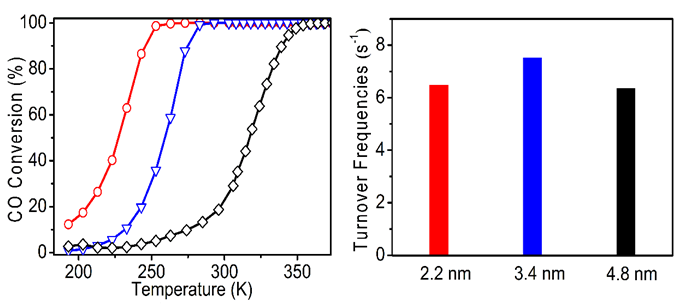
Colloid immobilization method to synthesis 2–5 nm Au sols and supported their on TiO2 supports. It was found that the activity of Au/TiO2 catalyst over CO oxidation decreased with the increase of the size of gold particles. Aberration corrected scanning transmission electron microscopy characterization was used to investigate geometric structure of Au particles and the Au-TiO2 interfacial perimeter. They calculated the turnover frequencies of the perimeter Au atom in the Au/TiO2 catalysts, with Au particle sizes of 2–5 nm, the value were quite similar. It has been demonstrated that the activity of Au/TiO2 catalysts could be related to the low-coordination sites of perimeter gold atoms. It is concluded that the size effect of gold particles in the range of 2-5 nm is origin from the bonding strength between Au-TiO2 at the interface and the dispersion of nanoparticles. This work is great significance to understanding of the size effect in nanocatalysis.
The research was supported by National Natural Science Foundation of China.(Text by Bin Shao)