Bimetallic nanoparticles catalyze hydrogenation of multiple carbon–carbon bonds actively and selectively. But accurately describing the atomic structure of the active sites is experimentally challenged by the averaging ensemble effect that is caused by the interplay between particle size and crystal-phase at elevated temperatures and under reactive gases.
Recently, our group in collaboration their partners, has quantified the geometric structure and the electronic character of the active sites over same-sized but crystal-phase-varied PdCu single-nanoparticles.
This study was published in Nature Communications on Aug. 5.
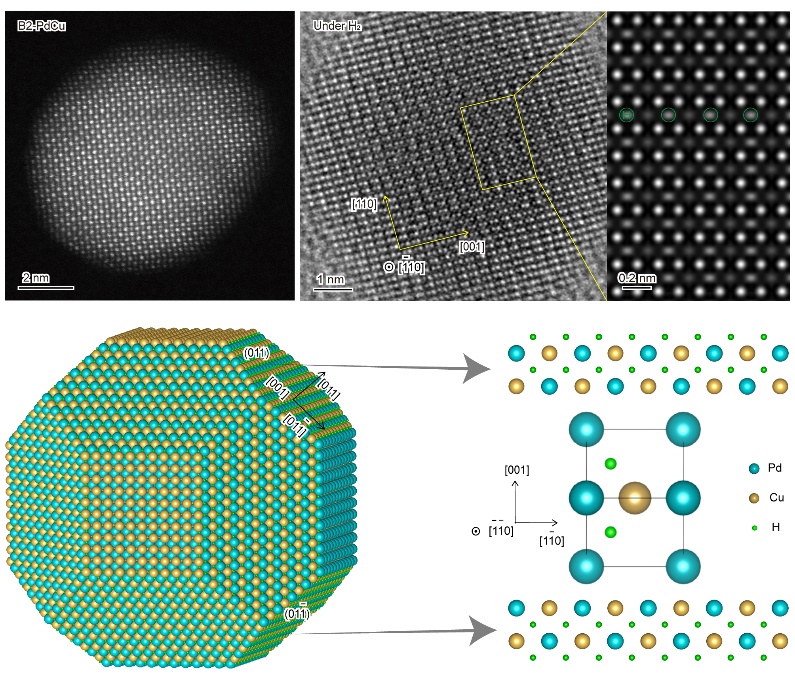
The HAADF-STEM images of a B2-PdCu particle in vacuum, exposed to H2 atmosphere and the simulated structure. (Image by LIU Shuang and HAN Shaobo)
The researchers first developed a strategy to tune the disordered and ordered phases of PdCu single-particle by keeping the particle size and chemical composition unchanged. The chemically ordered body-centered cubic (B2) particle was enclosed mainly by the {110} facets with an ordered arrangement of Pd and Cu atoms, while the disordered face-centered cubic (fcc) particle was terminated dominantly by the {111} facets where Cu and Pd atoms arranged randomly. They interestingly find that the intrinsic activity of the B2 particle, for acetylene hydrogenation, was one order of magnitude greater than that of the fcc counterpart.
They then identified the bonding environments of the surface metal atoms over the two particles using a combined approach of extended X-ray absorption fine structure spectroscopy, infrared spectroscopy and density functional theory calculations. The B2 particle featured densely-populated surface Pd-Cu bonds and possessed isolated Pd site with a lower coordination number and a high-lying metal d-band center. This not only greatly expedited H2 dissociation on the Pd atom but also effectively accommodated the activated H atoms on the particle top/subsurfaces.
They further resolved the dynamic behavior of the active sites during catalysis by performing environmental TEM experiments. H2 dissociation was observed to occur rapidly and the dissociated H atoms occupied the interstitial sites between Pd and Cu atoms on the B2-PdCu particle.
“This provides a new approach to study single-nanoparticle catalysis with a chemical reaction by accounting for the atomic configuration of the active site,” said Prof. SHEN.
This work was supported by the National Natural Science Foundation of China. (Text by LIU Shuang)